

 about
projects
people
publications
resources
resources
visit us
visit us
search
search
about
projects
people
publications
resources
resources
visit us
visit us
search
search
Quick Links
Recent Citations
Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules. Dodd DO, Mechaussier S et al. Science. 2024 Apr 26;384(6694):eadf5489.
Necroptosis blockade prevents lung injury in severe influenza. Gautam A, Boyd DF et al. Nature. 2024 Apr 25;628(8009):835–843.
Cryogenic electron tomography reveals novel structures in the apical complex of Plasmodium falciparum. Sun SY, Segev-Zarko L-a et al. mBio. 2024 Apr 10;15(4):e0286423.
Conformational ensemble of yeast ATP synthase at low pH reveals unique intermediates and plasticity in F1-Fo coupling. Sharma S, Luo M et al. Nat Struct Mol Biol. 2024 Apr;31(4):657-666.
Parental histone transfer caught at the replication fork. Li N, Gao Y et al. Nature. 2024 Mar 28;627(8005):890–897.
Previously featured citations...Chimera Search
Google™ SearchNews
October 30-31, 2023
Planned downtime: The Chimera and ChimeraX websites and associated web services will be unavailable Oct 30 8am PDT – Oct 31 11:59pm PDT.
April 19, 2023
Chimera production release 1.17.1 is now available, fixing an issue with 1.17 for Windows and Linux. See the release notes for details.
April 13, 2023
Chimera production release 1.17 is now available. Updating is required to keep using the tools that run Blast Protein, Modeller, and multiple sequence alignment with Clustal Omega or MUSCLE, as these will soon stop working in older versions. See the release notes for details.
Previous news...Upcoming Events

UCSF Chimera is a program for the interactive visualization and analysis of molecular structures and related data, including density maps, trajectories, and sequence alignments. It is available free of charge for noncommercial use. Commercial users, please see Chimera commercial licensing.
We encourage Chimera users to try ChimeraX for much better performance with large structures, as well as other major advantages and completely new features in addition to nearly all the capabilities of Chimera (details...).
Chimera is no longer under active development. Chimera development was supported by a grant from the National Institutes of Health (P41-GM103311) that ended in 2018.
Feature Highlight
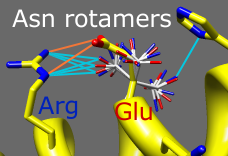
Amino acid sidechains adopt different conformational states, or rotamers. Rotamers from the Dunbrack backbone-dependent library or the Richardson "penultimate" library can be viewed, evaluated, and incorporated into structures with the Rotamers tool. A residue can be changed into a different conformation of the same type of amino acid or mutated into a different type. Rotamer torsion angles and library probability values are listed in a dialog, along with (optionally) hydrogen bonds, clashes, and agreement with electron density data. Only rotamers chosen in the list are displayed. When a single rotamer is chosen, it can be incorporated into the structure. The image includes 2D labels.
(More features...)Gallery Sample

Mutations that inactivate the tumor suppressor p53 are found in over 50% of human cancers, and most of the cancer-associated mutations are within its DNA-binding domain. The image shows a tetramer of the p53 DNA-binding domain complexed with DNA (Protein Data Bank entry 2ac0). The tetramer subunits are shown as light blue, green, orange, and yellow ribbons, with red spheres marking several major "hot spots" of mutation. The DNA is shown in purple and blue. (More samples...)
About RBVI | Projects | People | Publications | Resources | Visit Us
Copyright 2018 Regents of the University of California. All rights reserved.